Glioblastoma (GBM) is a highly lethal brain tumor with a high rate of relapse. Relapse is due to the presence within the tumor mass of a subset of cancer stem cells that are able to adapt, survive the stress induced by the microenvironment or anti-cancer therapy, and proliferate, supporting tumor regrowth. Therefore, interfering with cancer stem cells’ ability to adapt and survive may help in managing the disease.
LSD1 is a protein that plays a crucial role in modifying the gene activation/repression, involved in a number of physiological processes. In tumors, LSD1 is frequently overexpressed and promotes tumor growth. LSD1 inhibition, instead, hinders tumor growth and, for this reason, some LSD1 inhibitors are currently in clinical trials for lung cancer and leukemia.
In a paper recently published in Science Translational Medicine, by Faletti, Osti et al., researchers supervised by Giuliana Pelicci, director of the “Biology of Glioblastomas and Brain Metastases and Potential Therapeutic Targets” Unit, demonstrated, in preclinical models, the therapeutic potential of a specific drug that, by targeting LSD1 protein, was capable of limiting GBM growth.
The authors showed that, in vitro, LSD1 inhibitor (specifically, DDP_38003, which has shown benefits in AML mouse models) reduced LSD1 activity, as well as viability and growth of cancer stem cells specifically, while not affecting normal (neural progenitor) cells.
They also found that LSD1 protein was over-expressed in human GBM tissues as compared to normal human tissue, and, in particular, in the cancer stem cell subpopulation. Through in vivo experiments, they demonstrated LSD1 inhibitor ability to cross the blood-brain barrier, reach the brain, bind LSD1 and block its activity. While the treatment did not exert any significant adverse effect, it remarkably delayed tumor growth and prolonged mouse survival. Similar results were observed by reducing or completely eliminating LSD1 expression, further confirming that the effects were indeed specifically related to LSD1.
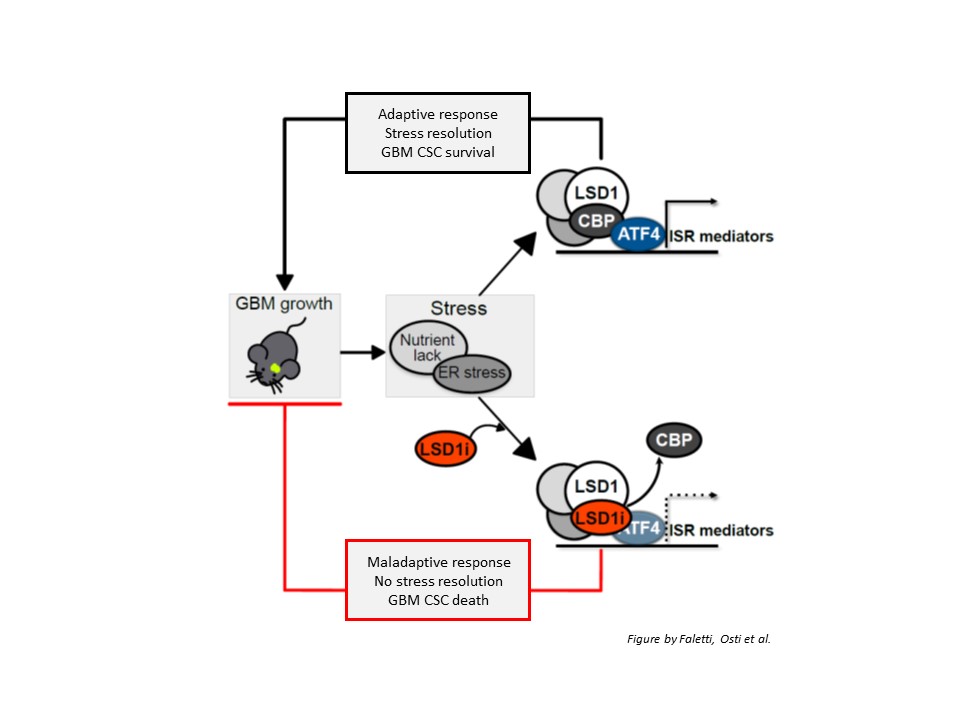
Next, the authors defined the underlying molecular mechanisms: LSD1 silencing down-regulated the expression of a number of genes involved in cells’ response to stress conditions (such as nutrient lack), namely the so-called integrated stress response (ISR); among them, ATF4, which is the main mediator of the ISR. Upon LSD1 inhibition, ATF4 activation was prevented, GBM stem cells were unable to properly handle stress conditions and died, resulting in reduced tumor growth. However, LSD1 or ATF4 re-expression was sufficient to restore cells’ ability to cope with stress and survive.
Importantly, LSD1 inhibitor acted by altering the interaction of LSD1 with CBP protein. The loss of LSD1/CBP interaction prevented ATF4 activation, thus making cancer stem cells unable to respond to stress conditions.
In conclusion, the authors provided convincing evidence supporting the potential of LSD1 as a novel therapeutic target in GBM: they showed that LSD1 inhibitor DDP_38003 is able to cross the blood-brain barrier, reach the brain and, by interacting with LSD1, prevent its binding to CBP protein, in turn impairing ATF4-dependent stress response. Since this mechanism is required to activate the program GBM stem cells need to survive stress conditions and proliferate, interfering with this process results in GBM stem cell death, thus likely contributing to reduce GBM growth and limit relapse.