Interorganelle communication in receptor signaling
Cellular organelles play a pivotal role in regulating signaling pathways by providing membrane platforms that facilitate the assembly of specific signaling complexes. The close proximity of organelles enables the formation of membrane contacts sites, allowing direct interorganelle communication. These contact sites help coordinate signaling and metabolic pathways through specialized membrane microdomains.
Our research has uncovered a critical link between EGFR endocytosis, interorganelle communication and signaling. EGFR is internalized through two main routes: clathrin-mediated endocytosis (CME) and non-clathrin endocytosis (NCE) (1-4). At low EGF doses (1–3 ng/ml), CME is activated, directing EGFR to recycling pathways and sustaining signaling.
In contrast, at high EGF doses (≥10 ng/ml) and in specific cellular contexts, NCE is activated, which targets EGFR for lysosomal degradation, leading to long-term signal attenuation.
EGFR-NCE relies on the formation tripartite contact sites between the plasma membrane (PM), the endoplasmic reticulum (ER), and mitochondria, mediated by the ER-resident protein Reticulon 3 (5). These tripartite contact sites are critical for PM invagination and the execution of EGFR-NCE. They serve as localized calcium signaling hubs, where Ca²⁺ released from the ER is taken up by nearby mitochondria. This calcium uptake influences mitochondrial function, stimulating the localized production of ATP in proximity of the PM, which is required for EGF-induced collective cell migration (6,7).

These observations provide a mechanistic framework to explain how EGF induces diverse cellular responses. At low concentrations, when CME is active, EGF acts as a potent mitogen. However, at high concentrations, when NCE is activated, EGF triggers a collective migratory response. Thus, through the activation of different endocytic routes, cells can decode a quantitative signal (i.e., EGF concentration) and translate it into distinct cellular responses.
Ongoing research aims to further elucidate the molecular mechanisms governing interorganelle communication in EGFR signaling and its relevance to physiological processes and cancer.
Endocytic dysregulation in cancer
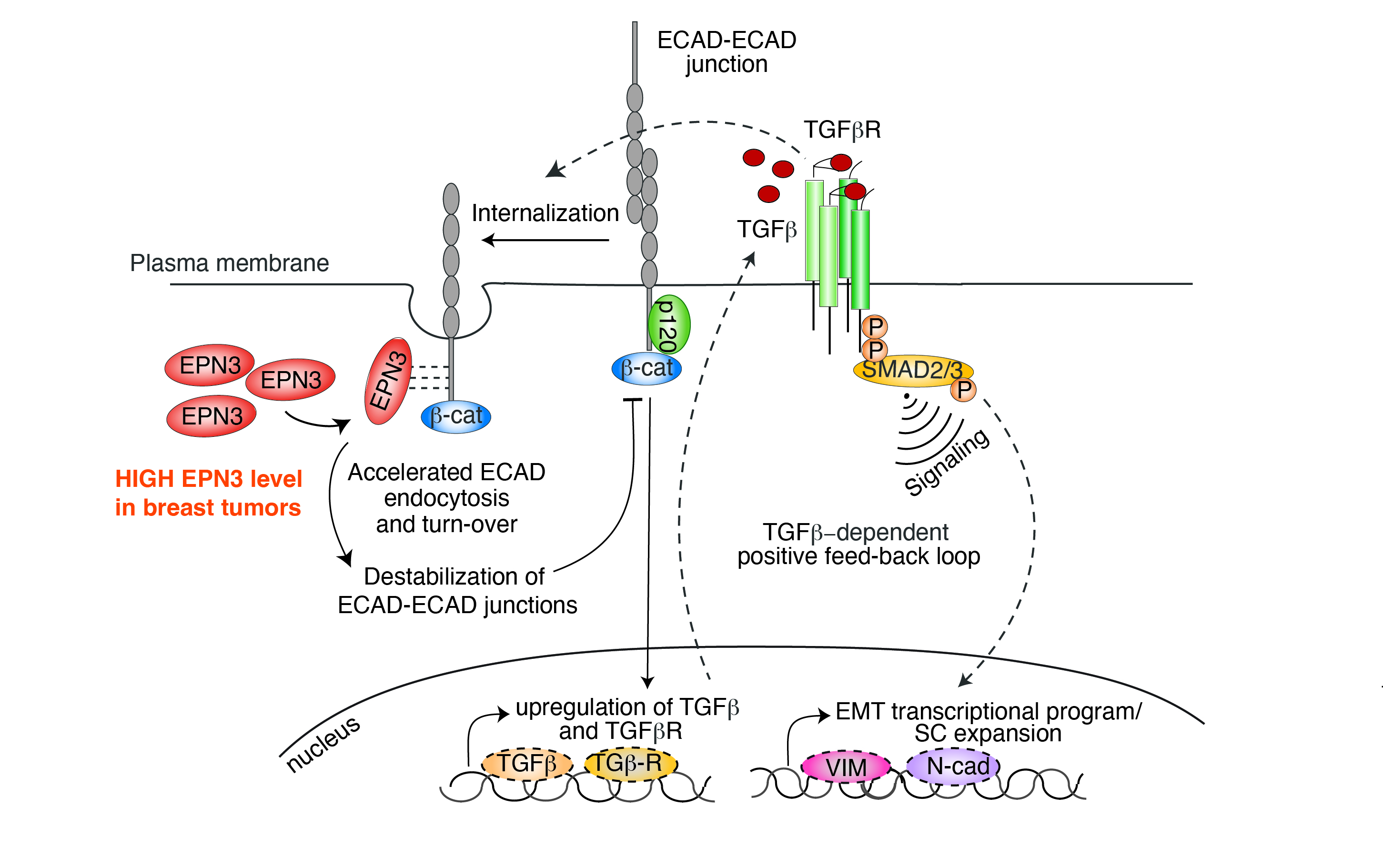
Subversion of endocytic genes can play an important role in cancer. Our research has shown that the endocytic adaptor protein Epsin3 (EPN3) is frequently overexpressed in breast cancer, either due to gene amplification or other mechanisms (8,9). Notably, EPN3 overexpression serves an independent predictor of poor prognosis, correlating with an increased risk of distant metastasis.
Mechanistically, we have established that EPN3 overexpression results in a partial epithelial-to-mesenchymal transition (pEMT) through an endocytic-based mechanism. Specifically, EPN3 overexpression increases E-cadherin endocytosis, leading to the destabilization of cell-cell junctions and the translocation of b-catenin to the nucleus. In turn, nuclear β-catenin activates a pEMT transcriptional program via a TCF4-dependent mechanism, establishing a TGFb-dependent autocrine loop that sustains EPN3-dependent phenotypes. This EPN3-induced circuitry leads to expansion of the cancer stem cell compartment, and promotes breast tumorigenesis and the transition from in situ to invasive breast cancer (8).
The EPN3 case study underscores the importance of endocytic dysregulation in cancer, demonstrating how alterations in endocytic pathways can drive cellular plasticity through the acquisition of a pEMT state. This, in turn, enhances the invasiveness and aggressiveness of breast cancer cells.
Ongoing research aims to further define the physiological and pathological roles of EPN3, with the ultimate goal of developing precision therapies for metastatic breast cancer.
References
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005 Feb 22;102(8):2760-5. doi: 10.1073/pnas.0409817102.
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008 Aug;15(2):209-19. doi: 10.1016/j.devcel.2008.06.012.
- Sigismund S, Algisi V, Nappo G, Conte A, Pascolutti R, Cuomo A, Bonaldi T, Argenzio E, Verhoef LG, Maspero E, Bianchi F, Capuani F, Ciliberto A, Polo S, Di Fiore PP. Threshold-controlled ubiquitination of the EGFR directs receptor. EMBO J. 2013 Jul 31;32(15):2140-57. doi: 10.1038/emboj.2013.149.
- Pascolutti R, Algisi V, Conte A, Raimondi A, Pasham M, Upadhyayula S, Gaudin R, Tanja Maritzen T, Barbieri E, Caldieri G, Freddi S, Confalonieri S, Malabarba MG, Maspero E, Polo S, Tacchetti C, Haucke V, Kirchhausen T, Di Fiore PP, Sigismund S. Molecularly distinct clathrin-coated pits differentially impact on EGFR fate and signaling. Cell Reports. 2019 Jun 4; 27(10):3049-3061.
- Caldieri, G, Barbieri, E, Nappo, G, Raimondi, A, Bonora, M, Conte, A, Verhoef, LGGC, Confalonieri, S, Malabarba, M G, Bianchi, F, Cuomo, A, Bonaldi, T, Martini, E, Mazza, D, Pinton, P, Tacchetti, C, Polo, S, Di Fiore, PP, Sigismund, S. Reticulon 3-dependent ER-PM contact sites control EGFR non clathrin endocytosis. Science 2017 May 12;356(6338):617-624. doi: 10.1126/science.aah6152.
- Mesa D, Barbieri E, Raimondi A, Freddi S, Miloro G, Jendrisek G, Caldieri G, Quarto M, Schiano Lomoriello I, Malabarba MG, Bresci A, Manetti F, Vernuccio F, Abdo H, Scita G, Lanzetti L, Polli D, Tacchetti C, Pinton P, Bonora M, Di Fiore PP, Sigismund S. A tripartite organelle platform links growth factor receptor signaling to mitochondrial metabolism. Nat Commun. 2024 Jun 15;15(1):5119. doi: 10.1038/s41467-024-49543-z.
- Sigismund S, Lanzetti L, Scita G, Di Fiore PP. Endocytosis in the context-dependent regulation of individual and collective cell properties. Nat Rev Mol Cell Biol. 2021 Sep;22(9):625-643. doi: 10.1038/s41580-021-00375-5. PMID: 34075221.
- Schiano Lomoriello I, Giangreco G, Iavarone C, Tordonato C, Caldieri G, Serio G, Confalonieri S, Freddi S, Bianchi F, Pirroni S, Bertalot G, Viale G, Disalvatore D, Tosoni D, Malabarba MG, Disanza A, Scita G, Pece S, Pilcher BK, Vecchi M, Sigismund S, Di Fiore PP. A self-sustaining endocytic-based loop promotes breast cancer plasticity leading to aggressiveness and pro-metastatic behavior. Nat Commun. 2020 Jun 15;11(1):3020. doi: 10.1038/s41467-020-16836-y.
- Giangreco G, Malabarba MG, Sigismund S. Specialised endocytic proteins regulate diverse internalisation mechanisms and signalling outputs in physiology and cancer. Biol Cell. 2021 Apr;113(4):165-182. doi: 10.1111/boc.202000129.