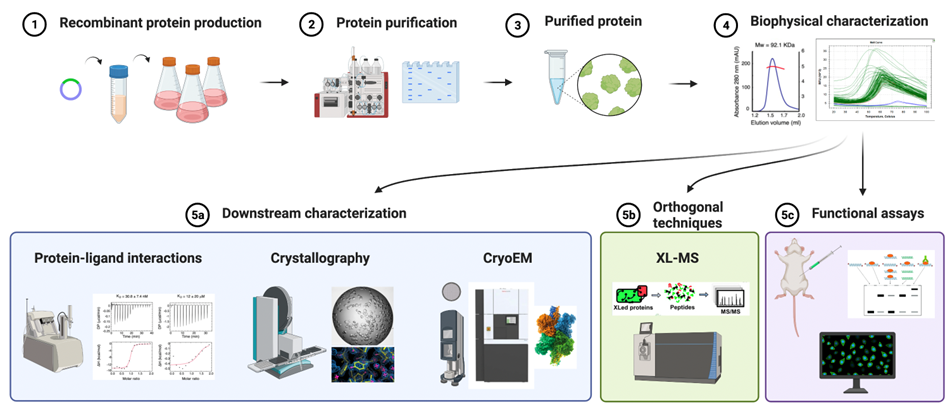
The Biochemistry and Structural Biology Unit (BSU) provides a wide range of services including recombinant protein production and purification, protein and protein complexes biophysical characterization and protein structural biology (X-ray crystallography and CryoEM).
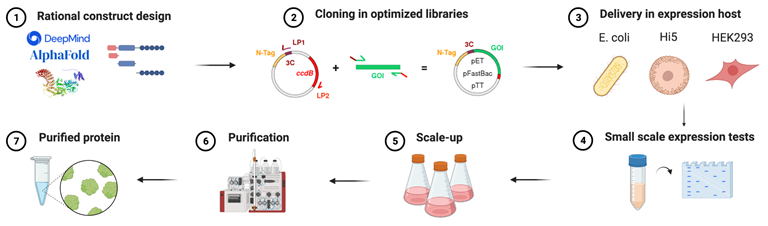
The BSU at IEO provides experienced assistance in project and constructs design prior to protein production. Using the latest software for protein and protein complexes predictions, we will provide and initial assessment of feasibility, guiding the user to the most promising protein design.
Cloning
We have a specifically designed plasmid library that is combined with SLIC (Sequence and Ligation Independent Cloning) system for a fast and efficient generation of expression plasmids containing the gene of interest. This allows to screen different expression hosts, tag types (His, STREP, etc) and location (N- or C-terminal) very efficiently.
Expression
The BSU is capable of protein production in bacteria (E. coli), insect cells (SF9 and Hi5) and mammalian cells (HEK293). We will guide the user to the selection of the appropriate expression system depending on the needs and protein target.
We can express proteins in E. coli using a variety of expression strains and many common fusion tags. Expression in bacteria is usually chosen for the recombinant production of small proteins or protein constructs that do not require post-translational modifications (PTMs).
We have an established pipeline for the recombinant production of proteins and protein complexes in insect cells using MultiBAC and GoldenBAC systems. These systems allow the simultaneous expression of up to 15 proteins, each one under its own promoter to normalize and increase overall protein complex yield.
For challenging targets requiring specific PTMs, transient transfection of HEK293 cells grown in suspension will be used.
Purification
The BSU makes use of a wide range of affinity beads to be used in gravity flow and columns for FPLC systems. Currently we have 3 AKTA purifier, 1 BioRad NGC system and 1 AKTA Xpress system operating in cell fridges at 4°C. For analytical purposes, we also have 2 AKTA micro systems operated with SEC columns and capillary for micro-fractionation.
Other services
The BSU assists users in several other protein production and purification services including antibody purification from sera using a strategy that combines protein A/G resin and affinity purification.
We also ensure the production of commonly used protein reagents such as Tn5 transposase, TAT-Cre recombinase, 3C protease and Cas9.
The BSU provides protein and protein complexes biophysical characterization.
We routinely assess protein quality using SEC-SLS (Seize-Exclusion Chromatography coupled with Static Light Scattering) that allows the characterization of protein monodispersity and the experimental determination of molecular weight.
The BSU can also provide experienced assistance in the characterization of protein stability using thermal shift assay under different buffer conditions to identify the most suitable condition for protein storage or further biophysical and structural studies.
Within our biophysics portfolio, we can measure binding affinities of protein-protein and protein ligand (DNA, peptides, small molecules) interactions using isothermal titration calorimetry (ITC – label free), fluorescence polarization (FP – label-based) and bio-layer interferometry (BLI – label free).
Crystallizing proteins is an art that requires perfect samples and high-throughput (and some luck). To maximize the chances of getting protein crystals the BSU has an automated platform for the high-throughput crystallization of macromolecular samples.
Initial trials are set up using Mosquito nanolitre-dispenser robot that allow to screen 96 conditions with as little as 15 μl of sample. Typically, we set-up at least 3-5 commercially available crystallization screen at 20° C and 4° C. We have a broad range of sparse and rational matrix screens covering the most common crystallization conditions. Crystallisation plates are automatically imaged on a standard schedule with the Bruker Crystal Farm 400, and drop images are available online, allowing remote monitoring of crystal appearance and growth.
Optimization of initial hits can be performed with robot in 96 well plates or manually in 24 well plates with the hanging or sitting drop techniques. In these cases, tailored optimization conditions are prepared based on the initial ones.
The BSU has routinely access to synchrotron light sources for crystal X-ray diffraction data collection to the European Synchrotron Radiation Facility (ESRF – Grenoble - FR) and Swiss Light Source (SLS - Villigen - CH) beamlines. Data are processed in-house with crystallographic software suites installed on a High-Performance Cluster.
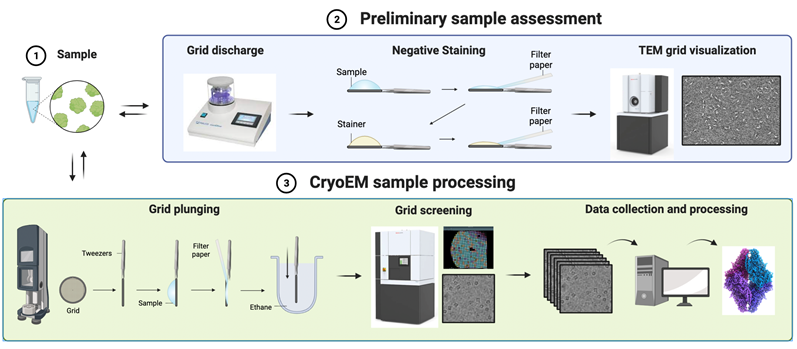
The BSU assists users in project design and cryoEM data processing services.
We are equipped with a glow discharge and a Vitrobot Mark IV for cryoEM grid preparation. Currently, we have access to a 200 kV screening microscope for grid screening and preliminary data collection. The most promising grids are sent to CM01 at the European Synchrotron Radiation Facility (ESRF – Grenoble - FR) for high-resolution data collection with a high-end 300 kV microscope. Data are processed in-house using the most updated softwares for cryoEM data processing installed on a dedicated multi-core and multi-GPU workstation.
For informal enquiries on our services please write here!